Last updated July 25, 2023
On July 24, HHS updated its Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV with an extesive secion on mpox which cover epidemioogy, prevention, clinicla presention, treatment, management of treatment failure, and special considerations for people with HIV
https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/mpox-monkeypox?view=full
The update also includes information on Chagas disease under the same link
Last updated May 19, 2023
202-690-6343
media@hhs.gov
Fact Sheet: HHS Provides Resources on Ways Communities Can Stay Protected from Mpox in Advance of Summer Months:
Last Summer, the United States faced an unprecedented outbreak of mpox with limited resources. The White House and the U.S. Department of Health and Human Services (HHS) launched an urgent, whole of government response to address the outbreak, which resulted in the deployment of tests, vaccines, and investigational treatments nationwide through HHS’ Administration for Strategic Preparedness and Response (ASPR).
Working in lock step with the LGBTQI+ community, this response resulted in significant declines in cases and the end of the mpox Public Health Emergency on January 31, 2023. As a result, today, the average daily case rate in the United States is 1 or fewer – over a 99% decline in daily case counts since the outbreak’s peak in August 2022.
As we head into the Summer, the Biden-Harris Administration is focused on increasing vaccine uptake to mitigate the risks of mpox and keep communities safe.
The United States stands in a strong position to prevent the expansion of this outbreak as we head into Spring and Summer 2023.
For months, the Biden-Harris Administration has been working closely with jurisdictions and partners to monitor trends, increase vaccine uptake, and improve vaccination rates in communities over-represented in the outbreak to keep them safe.
Since the earliest days of the mpox outbreak, we have not taken our foot off the gas in the fight against mpox and providing resources, education, and outreach to increase equitable access to vaccination, testing, and investigational treatments in communities that could benefit.
The mpox outbreak continues to be a public health priority for HHS and the Administration. For months, both have maintained outreach and education efforts and increased outreach in late winter 2022 and early Spring 2023 to motivate actions to prevent expansion of the current outbreak as festival and event seasons approach. Collectively, the Administration’s efforts aim to expand vaccination for individuals at risk and make testing more convenient for health care providers and patients across the country.
The Biden-Harris Administration remains committed to working with urgency to detect more cases, protect those at risk, and respond rapidly to new cases. As we head into the summer months, here’s important information and resources:
What We Know About the Virus:
Mpox is a disease that can cause flu-like symptoms and a rash. Based on currently available data, the vast majority of mpox cases in this outbreak have been associated with close skin-to-skin contact associated with sex between men.
Chicago has recently reported a new cluster of mpox cases after nearly three months with almost no mpox cases reported. Some of the cases are in people who have been vaccinated for mpox, and all are mild. The Centers for Disease Control and Prevention (CDC) is aware of these new cases and is working closely with Howard Brown Health, the Chicago Department of Health, and the local community to investigate these new cases and limit the size of this cluster.
How to Keep Yourself Safe:
Make sure that you receive two doses of the mpox vaccine if you are considered to be at-risk for mpox. If you only got one shot, it’s never too late to get the second dose.
If you are at risk for mpox but haven’t received your two-dose vaccine yet, temporarily changing some parts of your sex life might reduce the risk of exposure to the virus. Maintain those changes between your first and second shots of the vaccine since it takes two weeks after the second shot to achieve the highest protection. Knowing how mpox is transmitted allows you to make informed decisions about your sex life to further reduce your risk of exposure.
Seek health care and get tested if you have a rash, even if you have been previously vaccinated or had the infection. For more information on where to find testing, vaccines, or treatment, visit CDC’s mpox website.
What We Are Doing to Prevent Outbreaks:
Our recommendation ahead of the summer months is – “Get Healthy and Ready for Summer 2023” – by including mpox vaccination as part of a package of sexual health services that includes HIV and STI testing, treatment, and prevention.
A new mpox outbreak’s chances increase when fewer people are fully vaccinated. We encourage gay, men who have sex with men, and bisexual and transgender people who may be at risk for mpox exposure to get vaccinated or get their second dose if not fully vaccinated. It’s also important to remember that it is never too late to get the second dose. For information on where to find an mpox vaccine site near you, visit CDC’s mpox vaccine locator.
The mpox outbreak continues to be a public health priority for HHS and the Administration, and we will continue working to increase vaccine uptake to keep the outbreak under control.
Ensuring local health departments have the tools and resources they need to combat mpox and protect communities. The CDC, HHS, and White House are closely working with state, tribal, local, territorial public health departments, and other community partners to distribute vaccines where they are needed most.
For more information on the Administration’s equity-related outreach to communities nationwide, visit CDC’s mpox resources page.
Sharing data available on current mpox cases. Anyone, regardless of sexual orientation or gender identity, who has been in close, personal contact with someone who has mpox is at risk. Take steps to prevent getting mpox. If you have any symptoms of mpox, talk to a health care provider.
- For the latest information on current outbreak cases and data, visit CDC’s mpox Page.
- For the latest mpox digital resources – including posters, cards, stickers, and event organizer letters – visit CDC’s mpox Digital Resources Page.
- For the latest mpox communication toolkits – including toolkits specific to large gatherings, workplaces, and education programs – visit CDC’s mpox Community, Work & School Toolkits page.
Working with members of the LGBTQI+ community to prepare for summer Pride events. The White House and HHS have continued to engage with an extensive list of organizations and advocates. Early Spring 2023 outreach to prepare for festival season, beyond the routine weekly meetings used to inform public health leaders about mpox, has included the following organizations:
- InterPride
- CenterLink
- Center for Black Equity
- NMAC
- ASTHO
- APHL
- NAACHO
- ANAC
- CSTE
- BHOC
- Grindr
- AIDS United
- NGLCC
- GLMA
- GMHC
- NASTAD
- NCSD
- Health HIV
- BCHC
- LGBTQ Primary Care Alliance
- The Center for Black Health Equity
- Healthcare for the Homeless
- HUD, HRSA, CDC, and SAMSHA grantees
- The National Latinix Conference
- Members of the House and Ballroom Community
- The White House Mpox Equity Workshop
- Multiple LGBTQ focused event organizers
- HIVMA
Updated May 16
Potential Risk for New Mpox Cases
Distributed via the CDC Health Alert Network
May 15, 2023, 9:00 AM ET
CDCHAN-00490
Summary
In the United States, cases of mpox (formerly monkeypox) have declined since peaking in August 2022, but the outbreak is not over. The Centers for Disease Control and Prevention (CDC) continues to receive reports of cases that reflect ongoing community transmission in the United States and internationally. This week, CDC and local partners are investigating a cluster of mpox cases in the Chicago area. From April 17 to May 5, 2023, a total of 12 confirmed and one probable case of mpox were reported to the Chicago Department of Public Health. All cases were among symptomatic men. None of the patients have been hospitalized. Nine (69%) of 13 cases were among men who had received 2 JYNNEOS vaccine doses. Confirmed cases were in 9 (69%) non-Hispanic White men, 2 (15%) non-Hispanic Black men, and 2 (15%) Asian men. The median age was 34 years (range 24–46 years). Travel history was available for 9 cases; 4 recently traveled (New York City, New Orleans, and Mexico).
Although vaccine-induced immunity is not complete, vaccination continues to be one of the most important prevention measures. CDC expects new cases among previously vaccinated people to occur, but people who have completed their two-dose JYNNEOS vaccine series may experience less severe symptoms than those who have not.
Spring and summer season in 2023 could lead to a resurgence of mpox as people gather for festivals and other events. The purpose of this Health Alert Network (HAN) Health Update is to inform clinicians and public health agencies about the potential for new clusters or outbreaks of mpox cases and to provide resources on clinical evaluation, treatment, vaccination, and testing.
Background
A global outbreak of mpox began in May 2022. Previous outbreaks in places where mpox is not endemic were mostly related to international travel; however, this outbreak spread rapidly across much of the world through person-to-person contact, disproportionately affecting gay and bisexual men, other men who have sex with men (MSM), and transgender people. Most patients with mpox have mild disease, although some, particularly those with advanced or untreated HIV infection, may experience more severe outcomes.
As of May 10, a total of 30,395 cases have been reported in the United States. This outbreak had a peak of about 460 cases per day in August 2022, and gradually declined, likely because of a combination of temporary changes in sexual behavior, vaccination, and infection-induced immunity[1,2]. However, CDC continues to receive reports of new cases and clusters in the United States and internationally.
Although approximately 1.2 million JYNNEOS mpox vaccine doses have been administered in the United States since the beginning of the outbreak, only 23% of the estimated population at risk for mpox has been fully vaccinated. Vaccine coverage varies widely among jurisdictions. The projected risk of a resurgent mpox outbreak is greater than 35% in most jurisdictions in the United States without additional vaccination or adapting sexual behavior to prevent the spread of mpox [3]. Resurgent outbreaks in these communities could be as large or larger than in 2022.
To help prevent a renewed outbreak during the spring and summer months, CDC is urging clinicians to be on alert for new cases of mpox and to encourage vaccination for people at risk. If mpox is suspected, test even if the patient was previously vaccinated or had mpox. Clinicians should also refamiliarize themselves with mpox symptoms, specimen collection, laboratory testing procedures, and treatment options.
Recommendations for Clinicians Evaluating and Treating Patients
Conduct a thorough patient history to assess possible mpox exposures or epidemiologic risk factors. Mpox is usually transmitted through close, sustained physical contact and has been almost exclusively associated with sexual contact in the current global outbreak. It is important to take a detailed sexual history for any patient with suspected mpox.
Perform a complete physical examination, including a thorough skin and mucosal (e.g., oral, genital, anal) examination. Doing so can detect lesions of which the patient may be unaware.
Consider mpox when determining the cause of a diffuse or localized rash, including in patients who were previously infected with mpox or vaccinated against mpox. Differential diagnoses include herpes simplex virus (HSV) infection, syphilis, herpes zoster (shingles), disseminated varicella-zoster virus infection (chickenpox), molluscum contagiosum, scabies, lymphogranuloma venereum, allergic skin rashes, and drug eruptions. Specimens should be obtained from lesions (including those inside the mouth, anus, or vagina), if accessible, and tested for mpox and other sexually transmitted infections (STI), including HIV, as indicated. The diagnosis of an STI does not exclude mpox, as a concurrent infection may be present.
Patients with mpox benefit from supportive care and pain control. Mpox can commonly cause severe pain and can affect anatomic sites, including the anus, genitals, and oropharynx, which can lead to other complications. Assess pain in all patients with mpox virus infection and recognize that substantial pain may exist from mucosal lesions not evident on physical exam. Topical and systemic strategies should be used to manage pain. Pain management strategies should be tailored to the needs and context of an individual patient.
Tecovirimat is considered first-line among options that have not been approved by the U.S. Food and Drug Administration to treat eligible patients with mpox. If a clinician intends to prescribe oral tecovirimat, consider seeking access through enrollment in the AIDS Clinical Trials Group (ACTG) Study of Tecovirimat for Human Monkeypox Virus (STOMP) so that the trial can determine efficacy of this drug. This trial includes a placebo-controlled, randomized arm, and an open-label option for individuals with severe disease or those who decline randomization. Remote enrollment is available. For patients not eligible for the STOMP trial or who decline to participate, stockpiled oral tecovirimat is available upon request for mpox patients who meet treatment eligibility (e.g., have severe disease or are at increased risk for severe disease) under CDC’s Expanded Access Investigational New Drug (IND) protocol. More information about evaluating and treating patients can be found on the CDC mpox Clinical Guidance web pages.
Clinicians should notify their state or local health departments of any suspected or confirmed mpox cases (via 24-hour Epi On Call contact list).
Recommendations for Vaccinating Patients
JYNNEOS vaccine can be given as post-exposure prophylaxis (PEP) both to people with known or presumed exposure to the mpox virus. Vaccine can also be given to people with certain risk factors and recent experiences that may make them more likely to have been exposed to mpox. As PEP, vaccine should be given as soon as possible, ideally within 4 days of exposure; however, administration 4 to 14 days after exposure may still provide some protection against mpox. People who are vaccinated should continue to avoid close, skin-to-skin contact with someone who has mpox. JYNNEOS involves 2 vaccine doses given 28 days apart; peak immunity is expected 14 days after the second dose [4].
Previous studies have suggested that JYNNEOS vaccination is protective against mpox. When combined with other prevention measures, vaccination prior to exposure and PEP vaccination strategies might help control outbreaks by reducing transmission of the mpox virus, preventing disease, or reducing disease severity and hospitalization. Duration of immunity after one or two doses of JYNNEOS is unknown.
Currently, CDC does not recommend routine immunization against mpox for the general public. Mpox vaccination should be offered to people with high potential for exposure to mpox:
- People who had known or suspected exposure to someone with mpox.
- People who had a sex partner in the past 2 weeks who was diagnosed with mpox.
- Gay, bisexual, and other MSM, and transgender or nonbinary people (including adolescents who fall into any of these categories) who, in the past 6 months, have had
- A new diagnosis of one or more sexually transmitted diseases (e.g., chlamydia, gonorrhea, syphilis).
- More than one sex partner.
- People who have had any of the following in the past 6 months
- Sex at a commercial sex venue.
- Sex in association with a large public event in a geographic area where mpox transmission is occurring.
- Sex in exchange for money or other items.
- People who are sex partners of people with the above risks.
- People who anticipate experiencing any of the above scenarios.
- People with HIV infection or other causes of immunosuppression who have had recent or anticipate potential mpox exposure.
- People who work in settings where they may be exposed to mpox.
- People who work with orthopoxviruses in a laboratory.
Extensive risk assessment should not be conducted in people who request vaccination to avoid the barriers created by the stigma experienced by many who could benefit from vaccination. People in the community at risk (e.g., gay, bisexual, or other MSM; transgender or nonbinary people) asking for vaccination is adequate attestation to individual risk of mpox exposure. People who previously received only one JYNNEOS vaccine dose should receive a second dose as soon as possible.
For More Information
- Clinical Quick Reference
- Vaccination Basics for Healthcare Professionals
- Case Definitions for Use in the 2022 Mpox Response
- Clinical Recognition
- Clinical Considerations for Treatment and Prophylaxis of Mpox Infection in People Who are Immunocompromised
- Treatment Information for Healthcare Professionals
References
- Endo, A. et al. Heavy-tailed sexual contact networks and monkeypox epidemiology in the global outbreak. Science. 2022 Oct 7; 378 (6615):90-94. https://doi.org/10.1126/science.add4507
- Clay, P.A., et al. Modelling the impact of vaccination and behavior change on mpox transmission in Washington D.C. medRxiv (Preprint), 2023 Feb 14. Available at: https://doi.org/10.1101/2023.02.10.23285772
- CDC. Risk assessment of mpox resurgence and vaccination considerations. 2023 Apr 4. Available at: https://www.cdc.gov/poxvirus/mpox/response/2022/risk-assessment-of-resurgence.html
- Rao, A., et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:734-742. https://dx.doi.org/10.15585/mmwr.mm7122e1
Updated May 12, 2023
French officials recently posted an update on an Mpox cluster in the Center-Val de Loire region, with 17 cases reported since the first of the year, including 14 since March 1, 2023. All occurred in men who have sex with men who had several partners but did not attend any common events.
Five of the patients had received two Mpox vaccine doses in 2022. Also, five had received one smallpox dose during childhood, plus one dose in 2022.
Given the high proportion of vaccinated people in the cluster, 59%, Public Health France and its regional partners investigated the development, finding that the proportion of vaccinated cases is higher than the 25% observed at the national level between October 2022 and February 2023.
“It is appropriate to await the results of real-life efficacy studies which will allow better interpretation of these data. To date, there is little perspective on the efficacy of 3rd generation vaccines against Mpox infection,” Public Health France said in its statement.
In other Mpox developments, the European Centre for Disease Prevention and Control (ECDC) and the World Health Organization (WHO) European regional office yesterday posted a joint update on Mpox, which reported 28 new cases from 7 countries since the last update 4 weeks ago. Sixteen of the cases are part of the French cluster. Six were from Spain. Other countries reporting cases are Portugal, the Netherlands, Switzerland, Greece, and Malta.
https://www.cidrap.umn.edu/mpox/recent-french-mpox-cluster-includes-fully-vaccinated-patients
In the Unites States, the Chicago Department of Public Health released a health alert on May 9, 2023, with the following details:
- From April 17 to May 5, 2023, there were 12 confirmed and 1 probable case of Mpox reported, all among symptomatic men, at least 2 PLHIV
- 9 of 13 cases (69%) were among men who were fully vaccinated for Mpox; cases with previous vaccine had mild disease, 1 proctitis
- CDC has stated they have not identified clusters in other communities outside of Chicago at this time
Update February 28, 2023
MPOX at CROI 2023 – 7 minute read
MPOX IN PEOPLE LIVING WITH HIV AND CD4 COUNTS <350 CELLS/MM3: A
GLOBAL CASE SERIES
Conclusion: In our case series in PLWH with MPOX, severe systemic
complications and deaths occurred most commonly in persons with CD4 < 100
cells/mm3 and viraemia.
IMPACT OF VACCINATION ON MPOX INCIDENCE IN MSM ON PrEP IN THE
ANRS 174 DOXYVAC TRIAL
Conclusion: In France, MVA-BN vaccination in summer 2022 conferred
high-level protection against mpox infection in highly-at-risk MSM on PrEP.
In this study population, sexual behavior change did not seem to play a role in
reduction of mpox incidence.
HOUSEHOLD TRANSMISSION OF MPOX TO CHILDREN AND ADOLESCENTS
Conclusion: Among children with household contact to an adult with MPOX
in California, only 14% developed symptoms consistent with MPOX, and less
than 5% ultimately tested positive. The secondary attack rate may have been
underestimated because one-third of symptomatic children were not tested.
While the risk of household transmission is low, pediatric household contacts
should be offered post-exposure prophylaxis to prevent MPOX spread.
LONGITUDINAL ASSESSMENT OF VIRAL SHEDDING AMONG PATIENTS WITH
MPOX IN TORONTO, CANADA
Conclusion: Mpox virus genetic material may remain detectable in multiple
anatomic compartments for up to 8 weeks after symptom onset. Correlation
with infectivity requires further study.
MPOX DNA CLEARANCE IN SEMEN OVER SIX MONTHS FOLLOW-UP
Conclusion: These preliminary findings from this cohort of individuals
highlight that viral DNA clearance in seminal fluid samples from people
diagnosed with mpox infection was mostly observed within 2 weeks since first
positive test. These findings suggest that semen testing and prolonged use of
condoms after mpox infection may be necessary.
NEUTRALIZING AND T CELL RESPONSE AGAINST MPOX VIRUS AFTER
MVA-BN VACCINE
Conclusion: The first/single dose of MVA-BN triggers a humoral and cellular
response with nAbs response greater in primed vs. non-primed participants
independently of age. No evidence that HiSXV effect on nAbs response to
MVA-BN differed by HIV status. (Primed=historically small pix vaccinated)
COMPARISON OF SUBCUTANEOUS VERSUS INTRADERMAL ROUTE OF
ADMINISTRATION OF MVA VACCINE
Conclusion: MVA-BN was generally well tolerated; S-AEFIs were reported more
frequently by ID vaccine recipients as well as LSI-AEFI, apart from more frequent
local pain after SC. A larger increase in immunological markers was observed
with ID vs. SC administration, particularly for IgG and nAb. ID route proved to be
safe and immunogenic.
HUMORAL AND CELLULAR IMMUNE RESPONSE AFTER 3 MONTHS FROM
MPOX VIRUS INFECTION
Conclusion: Analysis of immune response after 3 months from MPXV infection
showed detectable IgG and nAb and increased CM, EM, and MVA-specific
responding T-cells, regardless of HIV infection, suggesting the possible
expansion of a protective memory/effector T-cells phenotype and the
persistence of immune protection.
IMMUNE RESPONSES AND VIRAL DYNAMICS AFTER MPOX INFECTION IN THE
2022 OUTBREAK
Conclusion: In our cohort, PWH with CD4+ >450/μL had a similar clinical
presentation of Mpox to HIVneg individuals. Magnitude of humoral immune
responses at the time of diagnosis was associated with a milder presentation
and a shorter and faster viral clearance of Mpox DNA in skin lesions. These
results may inform isolation strategies
NOVEL SEROASSAYS DETECT MPOX-SPECIFIC AND VACCINE-INDUCED
ORTHOPOXVIRUS IMMUNITY
Conclusion: We developed and validated the first mpox-specific seroassay
which uses the complete B21R peptide, which can distinguish recent infection
from vaccination, which in turn was associated with a robust E8L antibody
response. Collectively, our assays provide tools for conducting vaccine response
and immunosurveillance studies to longitudinally detect immunity to MPXV,
determine the true prevalence of MPXV infection and identify asymptomatic
community spread.
CLINICAL PREDICTORS OF MPOX SEVERITY IN AN ITALIAN MULTICENTER
COHORT (MPOX-ICONA)
Conclusion: We found that pts presenting with fever, facial/anal lesions, and
concurrent STIs may develop more severe Mpox. Moreover, higher VL in URT
during the first week after symptoms onset was associated with severe disease.
Our findings may serve to guide management of pts with Mpox in terms of need
for hospitalization and drug therapy. Finally, our study claims an urgent need
to assess whether the persistence of MPXV in biological samples after clinical
recovery may lead to a status of persistent infectivity.
SEVERE MPOX AMONG PEOPLE LIVING WITH HIV RECEIVING TECOVIRIMAT
IN NEW YORK CITY
Conclusion: This group of PWH with advanced HIV had severe mpox
manifestations and poor response to tecovirimat. Early and extended
tecovirimat with coadministration of other mpox treatments in the setting of
limited options is important to try to improve outcomes. Findings of severe
disease and high mortality highlight the urgency of mitigating deep social
inequities and high-quality research to optimize care in this group of PWH.
MANAGEMENT OF MPOX IN PWH ATTENDING A SEXUAL HEALTH
DEPARTMENT IN LONDON, UK
Conclusion: Despite low hospitalization rates in PWH with MPOX, medical
complications and STI rates requiring further management are significantly
high. Further comparative analysis with people without HIV and PWH with
severe immunodepression are needed to define risk factors for hospitalization
and clinical complications.
MOSAIC CLADE 2B MPOX COHORT STUDY: CLINICAL CHARACTERISATION
AND OUTCOMES
Conclusion: MOSAIC is an international study describing characteristics and
outcomes of Clade 2b Mpox; it does not support direct comparison between
tecovirimat-treated and non-treated patients. Lesions and symptoms resolved
within 28 days in most uncomplicated cases with supportive treatment without
hospitalisation. A higher proportion of patients presented with complications at
baseline in the tecovirimat-treated group. There was also a lower proportion of
patients in this group whose lesions had resolved with no serious complications
at D28.
DEVELOPMENT AND PILOT OF AN MPOX SEVERITY SCORING SYSTEM
(MPOX-SSS)
Conclusion: Our pilot MPOX-SSS was able to produce a severity score
retrospectively from 86% of charts, demonstrated good discrimination with
statistically higher scores in groups expected to have more severe disease, and
was able to distinguish change over time for individual patients that correlated
with clinical illness. We propose this tool be assessed for utility in clinical
trials of mpox treatment, in prospective observational cohort studies, and in
comparisons of illness caused by different mpox clades.
CLINICAL PRESENTATION OF MPOX IN PEOPLE WITH AND WITHOUT HIV
Conclusion: In this cohort of mpox cases there was a high prevalence of
well-controlled HIV co-infection, but we find no evidence that PLWH experience
more severe mpox.. Whilst there are a higher proportion of hospitalisations, this
is not statistically significant and is likely to be impacted by additional caution
shown by clinicians in making decisions around mpox care in these patients. All
other outcomes analysed indicate that mpox infections are of similar severity in
people with and without HIV, providing reassurance for patients and clinicians
providing future care for patients with mpox and HIV co-infection.
MPOX AMONG MSM IN THE NETHERLANDS PRIOR TO MAY 2022, A
RETROSPECTIVE STUDY
Conclusion: The first mpox cases in the Netherlands coincided with the
first cases reported in the United Kingdom, Spain and Portugal. We found no
evidence of widespread hMPXV transmission in Dutch sexual networks of MSM
prior to May 2022. Likely, the hMPXV outbreak expanded across Europe within
a short period in the spring of 2022 in an international highly intertwined
network of sexually active MSM.
CHANGES IN SEXUAL BEHAVIORS DUE TO MPOX: A CROSS-SECTIONAL
STUDY OF SGM IN ILLINOIS
Conclusion: SGM YYA in Illinois overwhelmingly reported reducing sexual
contact due to the mpox outbreak. Vaccinated individuals were more likely to
report sexual activity and a greater number of prophylactic activities. Thus, sexpositive
and harm reduction messaging strategies are likely to be more effective
than abstinence-only prevention, which may further stigmatize marginalized
groups.
STIGMA RELATED TO HUMAN MPOX VIRUS AMONG MSM IN THE US, AUGUST
2022
Conclusion: There was low overall prevalence of mpox-related stigma among
MSM in August 2022. These data suggest that messages developed by CDC and
others about mpox and how to protect oneself from mpox infection did not lead
to widespread stigma for this sample of MSM in the US.
HIGH LEVEL OF MPOX KNOWLEDGE AND STIGMA AMONG LGBTQIA+
COMMUNITIES IN BRAZIL
Conclusion: Our results show high rates of mpox knowledge in the LGBTQIA+
communities. Expand access to gender competent care is critical to avoid
underdiagnosis and fight stigma and discrimination.
CHARACTERISTICS AND DISPARITIES AMONG HOSPITALIZED PERSONS WITH
MPOX IN CALIFORNIA
Conclusion: Among persons with mpox and HIV, more hospitalized cases had
uncontrolled HIV and lived in communities with fewer opportunities to lead
healthy lives. Among persons with mpox and without HIV, more that were
hospitalized had diabetes or exfoliative skin disorders. Vaccination and rapid
access to testing and treatment should be prioritized in these groups.
MPOX VIRUS INFECTION IS MORE SEVERE IN PATIENTS WITH
UNCONTROLLED HIV INFECTION
Conclusion: PLWH, considered as a whole, are not at a greater risk of MPXV
severe disease. However, those with uncontrolled HIV infection, due to lack of
effective ART, develop more severe outcomes. Efforts should be done to increase
HIV testing and to ensure linkage to HIV care services. In this setting, ART must
be immediately started.
IMPACT OF HIV INFECTION ON MPOX-RELATED HOSPITALIZATIONS IN
BRAZIL
Conclusion: Our findings suggest an association between worse outcomes
in the HIV care continuum and mpox-related hospitalizations. Advanced
immunosuppression (CD4< 200) contributed to more severe clinical
presentations and death. Public health strategies to mitigate HIV late
presentation and the negative impact of the COVID-19 pandemic to the HIV care
continuum are urgently needed.
CHARACTERISTICS OF THE 2022 MPOX OUTBREAK IN A SOUTHEASTERN US
CITY
Conclusion: Clinical presentation of mpox in Atlanta was similar to other
reports; however, our cohort had a higher burden of HIV co-infection. Severe
mpox disease was observed at higher frequency in individuals with uncontrolled
HIV, indicating an urgent need to better understand the pathogenesis of
HIV-mpox interactions and to develop better prevention and treatment options
for PWH.
CLINICAL OUTCOMES AMONG IN- AND OUTPATIENTS WITH MPOX IN AN
URBAN HEALTH SYSTEM
Conclusion: In this multi-hospital system, a significant proportion of mpox
patients required hospitalization. Immunosuppression and HIV-1 viremia was
associated with hospitalization for mpox. Achieving viral suppression and mpox
immunization should be prioritized among those at risk.
HIV CARE AND PREVENTION CHARACTERISTICS AMONG PERSONS WITH
MPOX AND HIV, TEXAS 2022
Conclusion: Prevalence of HIV infection among persons with mpox was high,
similar to other findings. The majority of persons with mpox and HIV infection
were diagnosed with HIV more than 5 years ago and had HIV laboratory data
signifying utilization of HIV care services in the past year. The disproportionate
impact of mpox on those with HIV infection reinforces the importance of
offering HIV screening testing to persons seeking care for mpox and focusing
public health efforts on linkage or re-linkage to HIV care services as needed.
MPOX OUTBREAK IN PLWHA AND PrEP USERS IN A BRAZILIAN STI CENTER:
DIFFERENT CHALLENGES
Conclusion: The Mpox outbreak in Brazil curbed in September, possibly as a
result of the strong mobilization of the LGBTQIA+ community. The vast majority
of our study participants were PLWHA and PrEP users. PLWA in our study
presented more frequently with extragenital involvement than PrEP users,
possibly due to a weakened immune response of PLWHA to contain the spread to
distant areas. In low-incoming countries with limited diagnostic resources, the
development of an epidemiological and clinical screening prioritizing testing in
MSM, young ,with fever, adenomegaly and genital lesions, could be a strategy
to be implemented.
MPOX IN THE CONTEXT OF POPULATION-LEVEL HIV TREATMENT AND HIV
PrEP PROGRAMS IN BC
Conclusion: A high proportion of mpox cases in BC were prescribed HIV PrEP,
consistent with overlapping risk behaviour and eligibility criteria for HIV PrEP
with mpox transmission and vaccine eligibility. A smaller proportion of the more
heterogeneous HIV Tx clients was similarly affected by mpox. The decline in
mpox cases suggests a potential impact of mpox vaccine uptake and/or altered
client behaviour. Cases of concurrent diagnosis of HIV and mpox emphasize the
importance of screening for sexually transmitted infections, including HIV, in
persons being evaluated for mpox.
CHARACTERISTICS OF PATIENTS HOSPITALIZED WITH MPOX DURING THE
2022 US OUTBREAK
Conclusion: Mpox infection in the current U.S. outbreak has been associated
with severe morbidity and mortality, particularly among persons with AIDS. The
disproportionate burden of severe mpox among persons of color and persons
experiencing homelessness echoes inequities seen in the continuum of care
for PWH. Providers should test sexually active patients with suspected mpox
infection for HIV and other sexually-transmitted infections as indicated at the
time of mpox testing. Engaging all PWH in care remains a critical public health
priority, with additional efforts in HIV outreach and care retention needed to
reduce the population at risk for severe mpox.
EQUITY FOCUSED EVALUATION OF MPOX CARE METRICS IN KING COUNTY,
WA
Conclusion: Public Health and healthcare organizations rapidly scaled-up
mpox testing and treatment over the course of the 5-month epidemic allowing
for most patients to receive TPOXX without significant racial disparities. Testing
and treatment was largely dependent on a single sexual health clinic and
university-affiliated sites.
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF MPOX WITHIN A NEW
YORK CITY HEALTH SYSTEM
Conclusion: In a diverse cohort of mpox patients, treatment with tecovirimat
was well tolerated and associated with minimal adverse effects. The majority
of hospitalizations occurred in patients with underlying immunocompromising
conditions.
MPOX INFECTION IN WOMEN: A CASE SERIES FROM BRAZIL
Conclusion: We describe different epidemiological, behavior and clinical
profiles of mpox among women and men. The milder mpox clinical presentation
in women can be related to their lower HIV prevalence compared to men. Health
services must provide a comprehensive clinical and epidemiological assessment
that accounts for gender diversity to address the knowledge gaps regarding the
impact of mpox on both cisgender and transgender women.
Update February 17 2023
Fourth meeting of the WHO International Health Regulations Emergency Committee on mpox
Updated January 31
HIV.gov convened a meeting of federal HIV communications leadership to start the new year off with the critical message that integrating mpox messaging into our ongoing communications is foundational to our HIV response for 2023. While we recognize the important work that has been done to dramatically decrease new mpox cases, we cannot take our foot off the pedal, as there is still critical work needed to increase and routinize mpox vaccinations.
We were fortunate to hear about mpox in the context of HIV and the importance of an equity-centered response from White House and other U.S. Government leaders. Demetre C. Daskalakis, MD, MPH, Director, CDC Division of HIV/AIDS Prevention and Deputy Coordinator, White House National Mpox Response noted that mpox continues to be a public health issue that disproportionately impacts people with HIV, and data suggest that approximately 40% of people diagnosed with mpox in the United States also have HIV. He also highlighted important action steps (see below) and resource videos to move us forward.

Beginning the meeting, Kaye Hayes, MPA, Deputy Assistant Secretary for Infectious Disease and Director of the Office of Infectious Disease and HIV/AIDS Policy, emphasized the Biden-Harris Administration’s focus on the importance of equity in the mpox response to ensure that no communities are left behind. She also highlighted the intentionality of the work surrounding mpox, including hearing directly from populations most likely to be affected by mpox but least likely to be vaccinated to better understand what is working and what needs to be improved in our response.
Decline in Mpox Cases
During the meeting, Dr. Daskalakis noted that in the U.S., there has been around a 99% reduction in the number of daily mpox cases since the peak of the mpox outbreak in summer 2022. He attributed this to 1) effective communications to gay, bisexual, and other men who have sex with men, as well as transgender individuals and other gender-diverse individuals; 2) the mpox vaccine; and 3) swift response from the LGBTQI+ community.
Syndemics and Mpox
Dr. Daskalakis also discussed mpox in the context of syndemics, noting that mpox infection does not occur in isolation. The September 2022 CDC Morbidity and Mortality Weekly Report showed that HIV or recent sexually transmitted infections (STIs) are common among people with mpox. Dr. Daskalakis thus stressed the importance of continuing to ensure equitable access to mpox screening, prevention, and treatment, including both prioritizing people with HIV and STIs for mpox vaccination and offering HIV and STI screening for people evaluated for mpox. He also emphasized the need to encourage those who haven’t received their second vaccinations to do so.
“We cannot take mpox in isolation,” he asserted. “We need to put it in the context of the interacting epidemics and the interacting social determinants of health that make mpox worse, or that propel mpox transmission.”
The Way-Forward
Dr. Daskalakis discussed navigating the future of the domestic response to mpox. As of the time of the meeting, there were 1,152,073 U.S. mpox vaccines administered. To get to zero mpox cases, he noted we must focus on communications to increase mpox vaccinations and magnify our communications for mpox vaccine administration. [Note: imagery promoting this blog will include this statement]. He also encouraged increased engagement with partners via social media, as well as official government websites, such as HIV.gov.
Harold Phillips, Director, The White House Office of National AIDS Policy, offered a closing message to attendees. He noted that the mpox response was “a true demonstration of when we use and follow the data and the science and we center the approach with equity, we CAN make a difference.”
Stay up to date on mpox and view the CDC’s Mpox Vaccine Equity Toolkit and their Cases and Data page. Also watch and share HIV.gov’s mpox videos featuring Dr. Daskalakis answering 14 top mpox questions.
Updated January 23
Today, the CDC team updated resources related to mpox. These may be found below
New and Updated CDC Resources:
MMWR: Epidemiology of Human Mpox — Worldwide, 2018–2021 NEW
Strategies for Talking with Patients about Vaccinations for Mpox UPDATED
Autopsy and Handling of Human Remains of Patients with Mpox UPDATED
What’s New & Updated UPDATED
Additional Funding Resources:
Mpox Guidance for CDC Grant Recipients
Mpox Considerations for Sexual Health Services (Dear Colleague Letters)
HRSA: Use of Ryan White HIV/AIDS Program Funds for Mpox
HUD’s HOPWA (Housing Opportunities for Persons with AIDS)
SAMHSA: Dear Colleague Letter on Using SAMHSA Grant Resources for Mpox-related Activities
Recently Updated—In Case You Missed It:
MMWR: Mpox Cases Among Cisgender Women and Pregnant Persons — United States, May 11–November 7, 2022
Data and Analytics:
2022 U.S. Mpox Outbreak UPDATED
U.S. Map & Case Count UPDATED
U.S. Mpox Case Trends Reported to CDC UPDATED
Global Map & Case Count UPDATED
Mpox Cases by Age and Gender, Race/Ethnicity, and Symptoms UPDATED
Mpox Vaccine Administration U.S. Map UPDATED
Demographics of Patients Receiving TPOXX for Treatment of Mpox UPDATED
Additional CDC Resources:
Health Alert Network (HAN)—Severe Manifestations of Mpox
Mpox Vaccine Confidence Insights Report
Mpox Vaccine Equity Pilot Program
Science Brief: Detection and Transmission of Mpox Virus
Mpox Vaccination Program Provider Agreement
Clinician Outreach and Communication Activity (COCA) Call
Clinical Considerations for Treatment and Prophylaxis of Mpox Virus Infection in People with HIV
Additional Intradermal Administration Sites: JYNNEOS Vaccine
Video: How to Administer Intradermal Vaccine in Forearm, Deltoid, and Scapula
Videos on mpox recommendations and updates from CDC leadership and partners
Completing a Death Certificate in the Setting of Mpox
V-safe after Vaccination Health Checker for Mpox Vaccine
Mpox Toolkit for Correctional and Detention Facilities
Safer Sex, Social Gatherings, and Mpox
Strategies for Talking with Patients about Vaccinations for Mpox
CDC’s Vaccine Equity Efforts in the Peach State- The Atlanta Black Pride Story
Stories from the Mpox Response
CDC-INFO On Demand – Publications
Additional Partner Resources:
Mpox Vaccine Locator (mpoxvaxmap.org)
CDC/IDSA Clinician Call: Updates & Emerging Issues on COVID-19 and Mpox
ASPR: JYNNEOS Mpox Vaccine Distribution by Jurisdiction
ASPR: Operational Planning Guide
Study of Tecovirimat for Human Mpox Virus (STOMP)
FDA: Emergency Use Authorization Fact Sheet
HHS amends PREP Act declaration increasing workforce authorized to administer mpox vaccines
HHS: Public Readiness and Emergency Preparedness (PREP) Act Coverage for Mpox
HHS: Statement From HHS Secretary Becerra on mpox
HHS: U.S. Government Mpox Research Summary
HIV.gov: Addressing Mpox Holistically
HIV.gov: Mpox and People with HIV Videos
NIH: U.S. Clinical Trial Evaluating Antiviral for Mpox Begins
PREP Act Coverage Frequently Asked Questions for Mpox
SAMHSA: Anxiety and Stress Related to Mpox
The White House: A Comprehensive Summary of Federally-Funded Mpox Research Projects
The White House: Mpox Press Briefing (9/28/2022)
WHO: Clinical Management and Infection Prevention and Control of Mpox
WHO: WHO recommends new name for monkeypox disease
Published in MMRW today
HIV and Sexually Transmitted Infections Among Persons with Monkeypox — Eight U.S. Jurisdictions, May 17–July 22, 2022
Summary
What is already known about this topic?
In the current global monkeypox outbreak, HIV infection and sexually transmitted infections (STIs) are highly prevalent among persons with monkeypox.
What is added by this report?
Among 1,969 persons with monkeypox in eight U.S. jurisdictions, 38% had HIV infection, and 41% had an STI in the preceding year. Among persons with monkeypox, hospitalization was more common among persons with HIV infection than persons without HIV infection.
What are the implications for public health practice?
It is important to leverage systems for delivering HIV and STI care and prevention and prioritize persons with HIV infection and STIs for vaccination. Screening for HIV and other STIs and other preventive care should be considered for persons evaluated for monkeypox, with HIV care and HIV preexposure prophylaxis offered to eligible persons.
Monkeypox and HIV
New Study Documents the Frequent Detection of Monkeypox Virus DNA in Saliva, Semen, and other Clinical Samples
https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2022.27.28.2200503
Updated June 30, 2022
This week, the administration released an new fact sheet on Monkey pox. Key is the rollout of a national strategy for smallpox vaccination against monkeypox for people at risk. This follows a decision by Quebec Province to do the same with that province and Montreal being the epicenters of the current outbreak in North America
As part of the monkeypox outbreak response, the Biden-Harris Administration is launching a national strategy to provide vaccines for monkeypox for individuals at higher risk of exposure. The strategy aims to mitigate the spread of the virus in communities where transmission has been the highest and with populations most at risk. This plan distributes the two-dose JYNNEOS vaccine, which the Food and Drug Administration (FDA) approved for protection against smallpox and monkeypox in individuals 18 years and older determined to be at high risk for smallpox or monkeypox infection. States will be offered an equitable allotment based on cases and proportion of the population at risk for severe disease from monkeypox, and the federal government will partner with state, local, and territorial governments in deploying the vaccines.
The goal of the initial phase of the strategy is to slow the spread of the disease. HHS will immediately allocate 56,000 vaccine doses currently in the Strategic National Stockpile to states and territories across the country, prioritizing jurisdictions with the highest number of cases and population at risk. To date, vaccines have been provided only to those who have a confirmed monkeypox exposure. With these doses, CDC is recommending that vaccines be provided to individuals with confirmed monkeypox exposures and presumed exposures. This includes those who had close physical contact with someone diagnosed with monkeypox, those who know their sexual partner was diagnosed with monkeypox, and men who have sex with men who have recently had multiple sex partners in a venue where there was known to be monkeypox or in an area where monkeypox is spreading.
In the coming weeks, HHS expects to receive an additional 240,000 vaccines, which will be made available to a broader population of individuals at risk. HHS will hold another 60,000 vaccines in reserve.
HHS expects more than 750,000 doses to be made available over the summer. An additional 500,000 doses will undergo completion, inspection, and release throughout the fall, totaling 1.6 million doses available this year.
First Case Report of Monkeypox in a Person Living with HIV
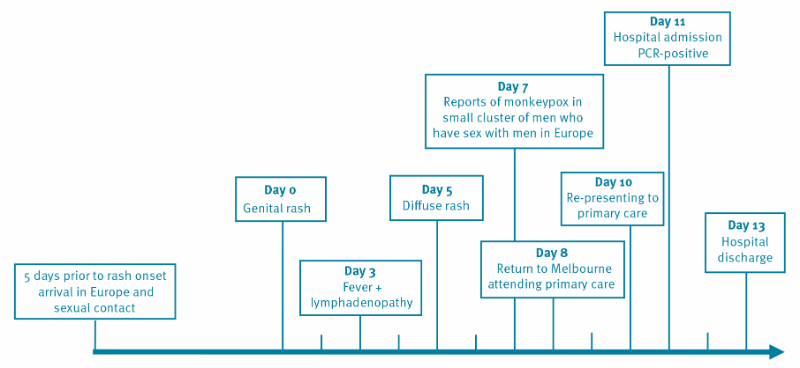
An HIV-positive man in his 30s taking Abacavir, Lamivudine and Dolutegravir and with a CD4 + T-cell count above 700 cells/mm3 (normal range 410–1,545 cells/mm3) and HIV viral load < 100 copies/mL, visited a primary care doctor after his return from Europe to Melbourne, Australia. He reported onset of a genital rash 8 days earlier. The rash had started 5 days after he reported unprotected sex with four casual male partners in Europe. The initial symptoms were painless white pustules on the penis that became painful and pruritic. He reported that he developed a fever and malaise 3 days after the first appearance of the penile rash and over the subsequent 5 days, the rash disseminated to his trunk, then more sparingly to the face and limbs while the genital lesions crusted over.
Swabs taken from deroofed skin lesions on the hand, calf and trunk in addition to combined nose throat swabs on the day of hospital admission, were all positive for monkeypox virus using previously described conventional [2] and in-house RT-PCR assays for orthopox and monkeypox viruses. Whole genome sequencing performed as described in the Supplementary material of DNA derived from the skin lesions resulted in the complete recovery of the entire monkeypox genome (MPXV-VIDRL01, Genbank_ID ON631963) with phylogenetic analysis revealing clustering with other monkeypox virus sequences from the May 2022 outbreak in Europe and the United States.
Rapid communication Home Euro surveillance Volume 27, Issue 22, 02/Jun/2022 Monkeypox infection presenting as genital rash, Australia Yael Hammerschlag et al
|
The World Health Organization (WHO) reported in its May 30, 2022, update that it had received reports of 257 confirmed monkeypox cases and approximately 120 suspected cases in 23 countries where the virus is not endemic as of May 26, 2022. In the United States, the Centers for Disease Control and Prevention (CDC) has reported 12 cases in eight states as of May 27, 2022. No deaths have been reported in nonendemic countries. The WHO classifies the global public health risk level posed by monkeypox as moderate. |
Background
Monkeypox was first detected in 1958 in laboratory monkeys.1 The first human case of monkeypox was recorded in 1970 in the Democratic Republic of Congo.2 Since then, monkeypox has been reported in humans in other central and western African countries, with occasional cases reported outside of Africa.1
Global Outbreak
In May 2022, more than 120 confirmed or suspected cases of monkeypox have been reported in at least 11 non-African (endemic) countries, including Australia, Belgium, Canada, England, France, Germany, Israel, Italy, Netherlands, Portugal, Spain, Sweden, and Switzerland.2
Historical Context
In 2003, the first monkeypox outbreak outside of Africa was in the United States, when 70 cases in humans were reported, linked to contact with infected pet prairie dogs, which had been housed with Gambian pouched rats imported into the United States from Ghana.3 Monkeypox was reported in travelers from Nigeria to the United States in July 2021 and November 2021.3
2022 US and Global Outbreak
On May 20, 2022, the US Centers for Disease Control and Prevention (CDC) issued an alert urging doctors and state health departments to be vigilant for cases of monkeypox, following confirmation of cases in the US.4, 5 Federal officials say they expect to identify additional infections in the coming days. According to the CDC, it is not clear how people in the cluster outbreaks so far were exposed to the monkeypox virus but cases include people who self-identify as men who have sex with men.6 Public health officials have issued similar alerts in Australia, Belgium, Canada, England, France, Germany, Israel, Italy, Netherlands, Portugal, Spain, Sweden, and Switzerland.
Monkeypox virus is known to spread through close contact with the lesions, bodily fluids and respiratory droplets of infected people or animals or materials contaminated with the virus. Human transmission is thought to occur primarily through respiratory droplets. Investigations are ongoing that the virus may be spreading by sexual contact, following outbreaks of monkeypox in Europe related to two parties in Spain and Belgium, attended primarily by gay men. Although many cases have been reported among men who have sex with men (MSM), and bisexual men, spread may be occurring because the virus was introduced into the community and it has continued to spread there, both by sexual and social contact.
Key Facts about Monkeypox3
- Monkeypox is caused by monkeypox virus.
- Monkeypox typically presents clinically with fever, rash, and swollen lymph nodes and may lead to a range of medical complications.7
- The incubation period is usually 7-14 days but can range from 5-21 days.
- Monkeypox is usually a self-limited disease with the symptoms lasting from 2 to 4 weeks. Severe cases can occur and the fatality ratio has been around 3-6%.
- Monkeypox is transmitted to humans through close contact with an infected person (skin lesions, body fluids, respiratory droplets and contaminated materials such as bedding) or animal, or with material contaminated with the virus.
- The clinical presentation of monkeypox resembles that of smallpox but is less contagious than smallpox and causes less severe illness.
- Vaccination against smallpox was demonstrated through several observational studies to be about 85% effective in preventing monkeypox. A vaccine based on a modified attenuated vaccinia virus (Ankara strain) was approved for the prevention of monkeypox in 2019.
- An antiviral agent (tecovirimat) that was developed for smallpox was licensed by the European Medical Association (EMA) for monkeypox in 2022 based on data in animal and human studies. Tecovirimat is not yet widely available.
Summary of CDC Recommendations for Clinicians6
- If clinicians identify patients with a rash that could be consistent with monkeypox, especially those with a recent travel history to areas reporting monkeypox cases, monkeypox should be considered as a possible diagnosis.
- The rash associated with monkeypox involves vesicles or pustules that are deep-seated, firm or hard, and well-circumscribed
- Presenting symptoms typically include fever, chills, the distinctive rash, or new lymphadenopathy; however, onset of perianal or genital lesions in the absence of subjective fever has been reported.
- Information on infection prevention and control in healthcare settings is provided on the CDC website’s Infection Control page.8
- Clinicians in the United States should consult their state health department or CDC through the CDC Emergency Operations Center (770) 488-7100 as soon as monkeypox is suspected.
- Clinicians outside of the United States consult their relevant subnational and national public health authorities for guidance and epidemiological surveillance purposes.
What At-Risk Individuals Should Do6
The CDC advises people who may have symptoms of monkeypox should contact their healthcare provider. This includes anyone who:
- Traveled to central or west African countries, parts of Europe where monkeypox cases have been reported
- Reports contact with a person with confirmed or suspected monkeypox
The World Health Organization (WHO) notes that available evidence suggests that those who are most at risk are those who have had close physical contact with someone with monkeypox, and that risk is not limited to men who have sex with men.
Notes
[1] Monkeypox goes global: why scientists are on alert Max Kozlov Nature News May 20 2022
[2] CDC Monkeypox https://www.cdc.gov/poxvirus/monkeypox/index.html Last updated May 20 2022
[3] Monkeypox World Health Organization May 19,2022 https://www.who.int/news-room/fact-sheets/detail/monkeypox
[4] CDC tells doctors to be on alert for monkeypox as global cases rise Washington Post https://www.washingtonpost.com/health/2022/05/20/cdc-monkeypox-alert/
[5] Monkeypox in the United States CDC https://www.cdc.gov/poxvirus/monkeypox/outbreak/us-outbreaks.html
[6] 2022 United States Monkeypox Case https://www.cdc.gov/poxvirus/monkeypox/outbreak/current.html
[7] Signs and Symptoms CDC https://www.cdc.gov/poxvirus/monkeypox/symptoms.html
[8] Precautions to Prevent Monkeypox Transmission https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-hospital.html







